Pipeline & Targets
Novel oncology & infectious disease medicines supported by multiple pharma partners & Federal government.
- Target strategy: Important targets that have been challenging to drug due to chemistry limitations that we can fix with our platform
- Partnering strategy: Partnerships are key to our success providing funding, investment, access to technology, and external validation
* This project has been funded in whole or in part with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Contract No. 75N93022C00060
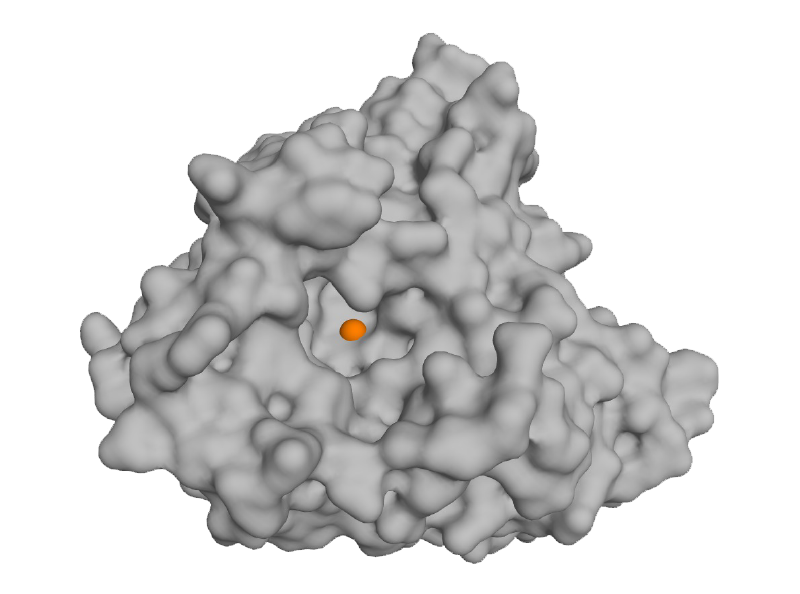
LpxC
LpxC, a zinc hydrolase, is an attractive and highly sought-after antibiotic target – it is conserved across Gram-negative bacteria and not found in Gram-positive bacteria or human cells. Inhibiting LpxC results in potent killing of Gram-negative bacteria with the benefit of sparing Gram-positive bacteria such as those residing in the protective microbiome of the gut which help to deter opportunistic C. difficile infections.
Other LpxC inhibitors have been evaluated by biopharma in the past but chemistry limitations (e.g.hydroxamic acid) have yielded ineffective compounds that suffer from poor drug-like properties. Thus, there are no approved therapeutics targeting LpxC. Blacksmith, using its proprietary chemistry platform, has developed novel non-hydroxamate inhibitors of LpxC that are safe and effective in animal models of Gram-negative infection and are able to kill Gram-negative ‘superbugs’ where other antibiotics are ineffective.
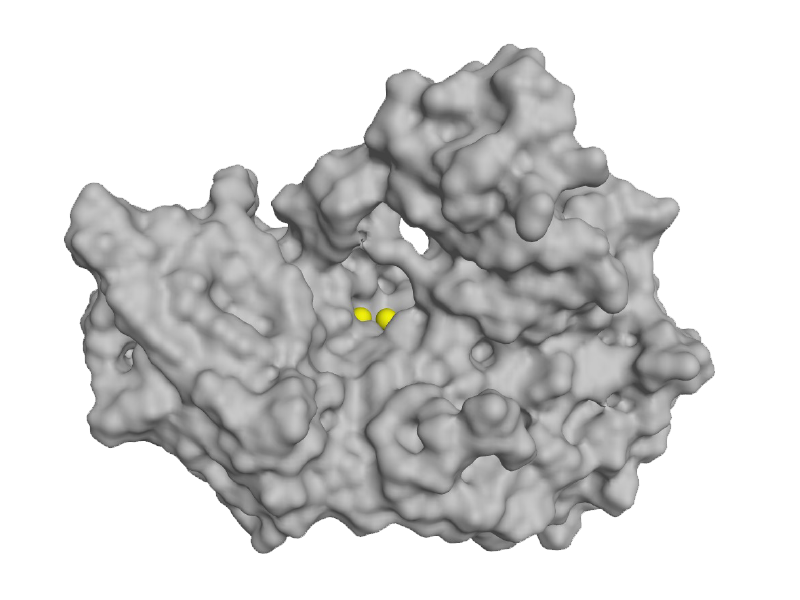
FEN1
Flap endonuclease 1 (FEN1) is a structure-specific di-magnesium metallonuclease that cleaves 5’ DNA flaps during replication and repair. FEN1 is an attractive target for development of anticancer therapeutics because it is overexpressed in many tumor types and has a large number of synthetic lethality partners including genes in Homologous Recombination (HR) pathway.
Other FEN1 inhibitors have been evaluated by biopharma in the past but chemistry limitations (e.g.hydroxyurea) have yielded ineffective compounds that suffer from poor drug-like properties. Thus, there are no approved therapeutics targeting FEN1. Blacksmith, using its proprietary chemistry platform, has developed novel non-hydroxyurea inhibitors of FEN1 that are potent and selective with promising ADME and PK properties.
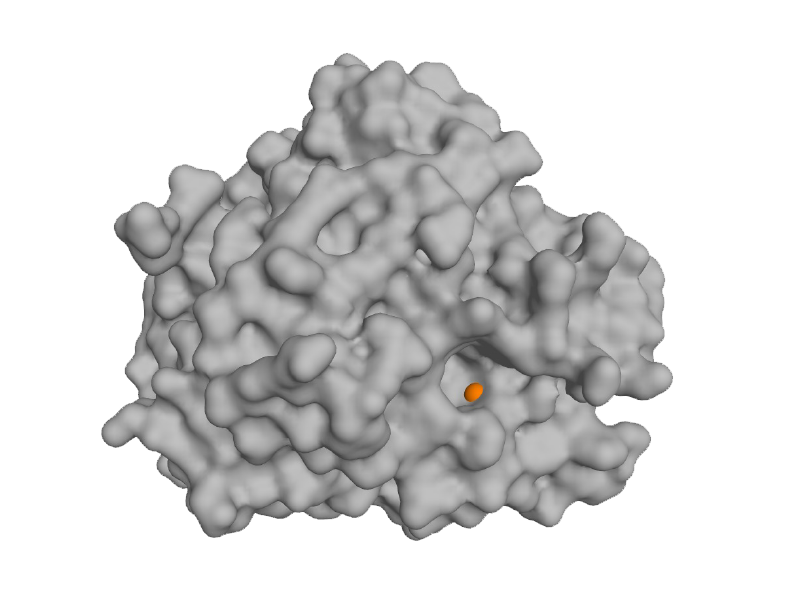
QPCTL
Glutaminyl-peptide cyclotransferase-like (QPCTL) is a metalloenzyme responsible for modifying the amino-terminus of the CD47 protein. The interaction between CD47 and Signal-regulatory protein alpha (SIRPa) delivers a “don’t eat me” signal to myeloid cells, constituting an innate immune checkpoint. Another substrate of QPCTL that undergoes N-terminal cyclization is the pro-tumorigenic chemokine CCL2. This chemokine recruits immunosuppressive cells to the tumor microenvironment and mediates pathological angiogenesis.
Inhibition of QPCTL presents a promising strategy for myeloid-cell-targeted cancer immunotherapy. However, no approved therapeutics currently exist, underscoring the need for innovative chemistry approaches. Leveraging a fragment-based drug discovery platform and expertise in metalloenzymes, Blacksmith has identified a series of compounds that demonstrate potency both in vitro and in vivo.
